

Many healthcare systems around the world struggle to cope with the often complex and challenging needs of an ageing population. While Qatar's population remains relatively young, the Ministry of Public Health (MOPH) recognized the need for strate..
.jpg)
According to the Ministry of Education and Higher Education, all staff and students in government and private nurseries, kindergartens, and schools must take the Rapid Antigen Test at home or one of the designated testing centers no later than 48 hou..
.jpg)
It has been said that Novartis, one of the most successful drug companies in the world, will soon start a marketing campaign called "Women in Pink." Because of this program, the breast cancer death rate will decrease in the Gulf region. ..
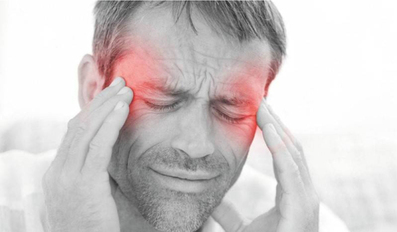
The Health Council of the Gulf Cooperation Council (GHC) has clarified that if a headache appears on one side of an individual's head, this does not mean that he suffers from a specific disease. The GHC's clarification came through its off..
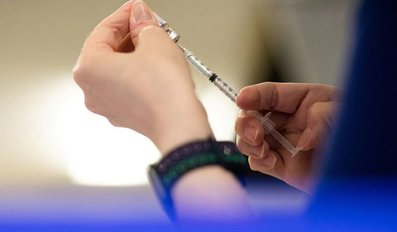
People at high risk of severe disease who have yet to get a second COVID-19 booster should not wait for next-generation, Omicron-targeted vaccines expected in the fall, five vaccine experts told Reuters. In many countries, including the United Sta..
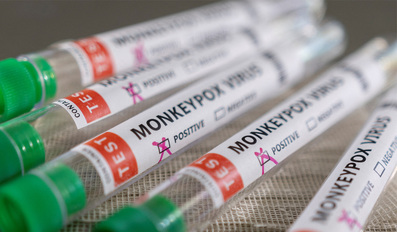
The Ministry of Public Health today announced the registration of the first confirmed case of monkeypox in the State of Qatar. The case was detected in a traveler returning to Qatar from abroad. The patient has been placed in hospital isolation ..
The monkeypox outbreak has been declared a global health emergency by the World Health Organization. The classification is the highest alert that the WHO can issue and follows a worldwide upsurge in cases. It came at the end of the second meeti..

Aiming to deliver quality healthcare services at affordable prices to all people in Qatar, Reyada Medical Centre has opened its doors at C-Ring Road with a soft launch. Reyada Medical Centre, a private medical service provider in Qatar, annou..

The Ministry of Public Health (MoPH) recently published a circular that addressed all private health facilities in Qatar. It discussed the following: • Circulation of pharmaceutical products within health facilities • Significanc..
.jpg)
Qatar Secures Place Among the World's Top 10 Wealthiest Nations
.jpg)
Hamad International Airport Witnesses Record Increase in Passenger Traffic

Saudi Arabia: Any visa holder can now perform Umrah

What are Qatar's Labour Laws on Annual Leave?