
BioNTech
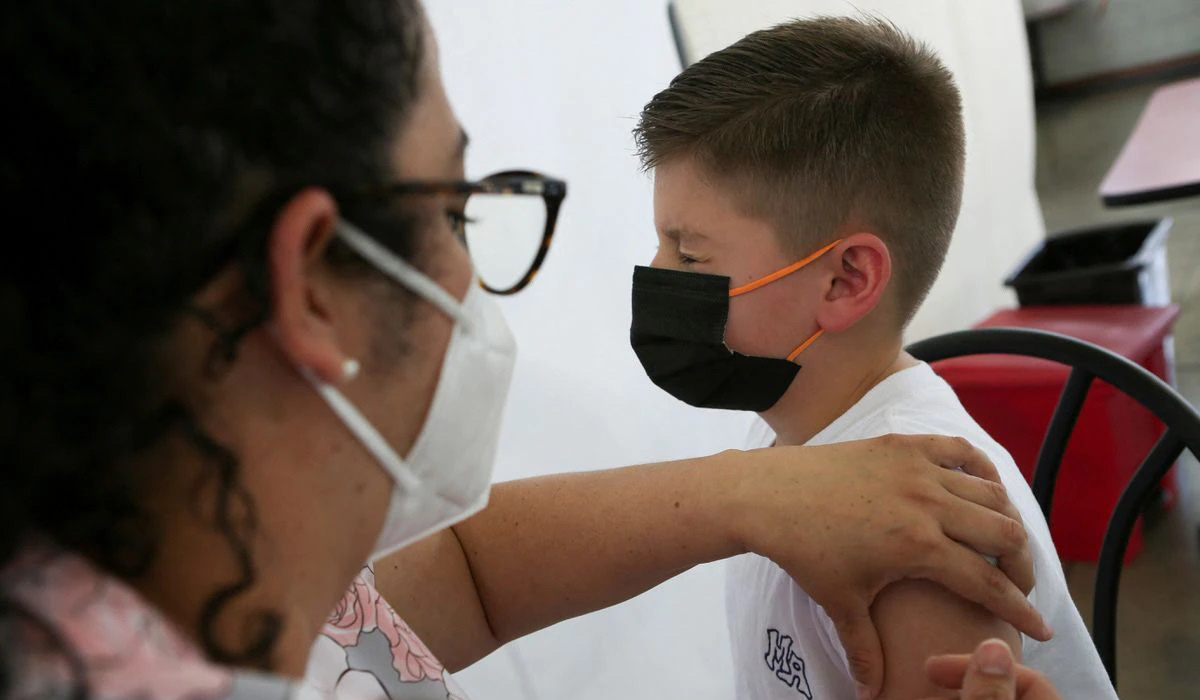
A third dose of the COVID-19 vaccine from Pfizer Inc (PFE.N) and BioNTech (22UAy.DE) produced significant protection against the Omicron variant of the coronavirus in healthy children ages 5 to 11, the companies said on Thursday. ..

New COVID variants inevitable, Ugur Sahin warns, but the world is ‘learning more’ and getting a ‘better understanding on how to deal with the virus’ The world is becoming “better prepared” to deal with futur..
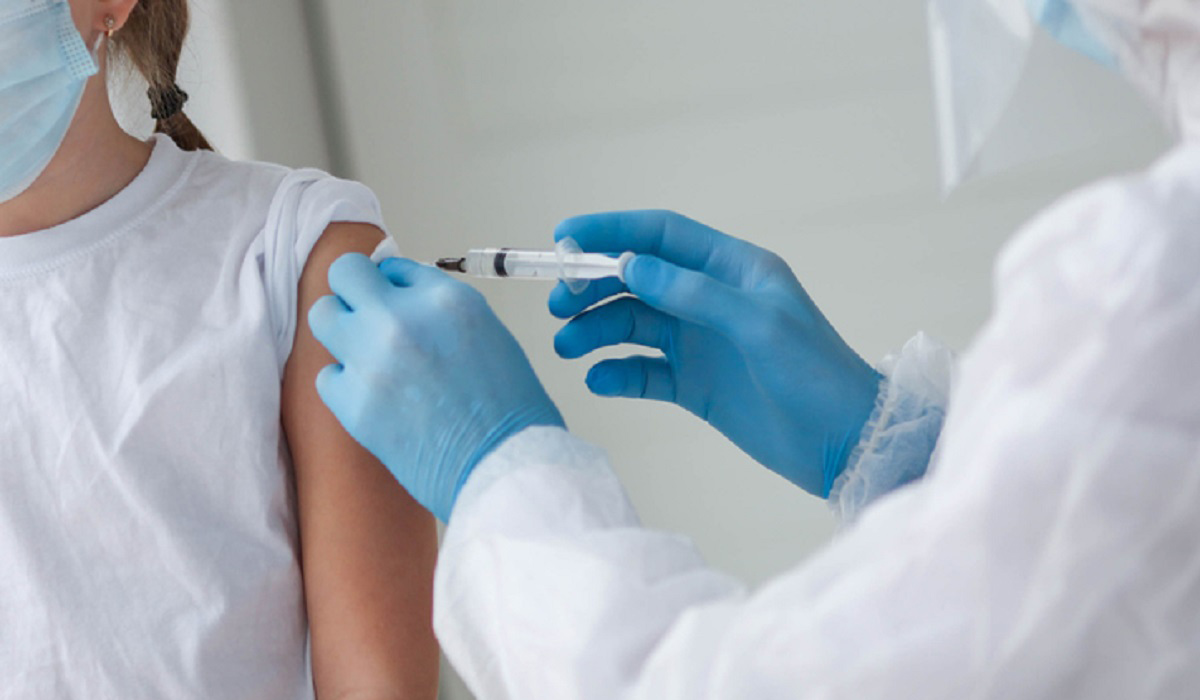
The Ministry of Public Health has approved the administration of Pfizer-BioNTech COVID-19 vaccines for children aged 5 to 11 years. The decision is in line with the recent studies that demonstrate the safety and efficacy of the Pfizer-BioNTe..
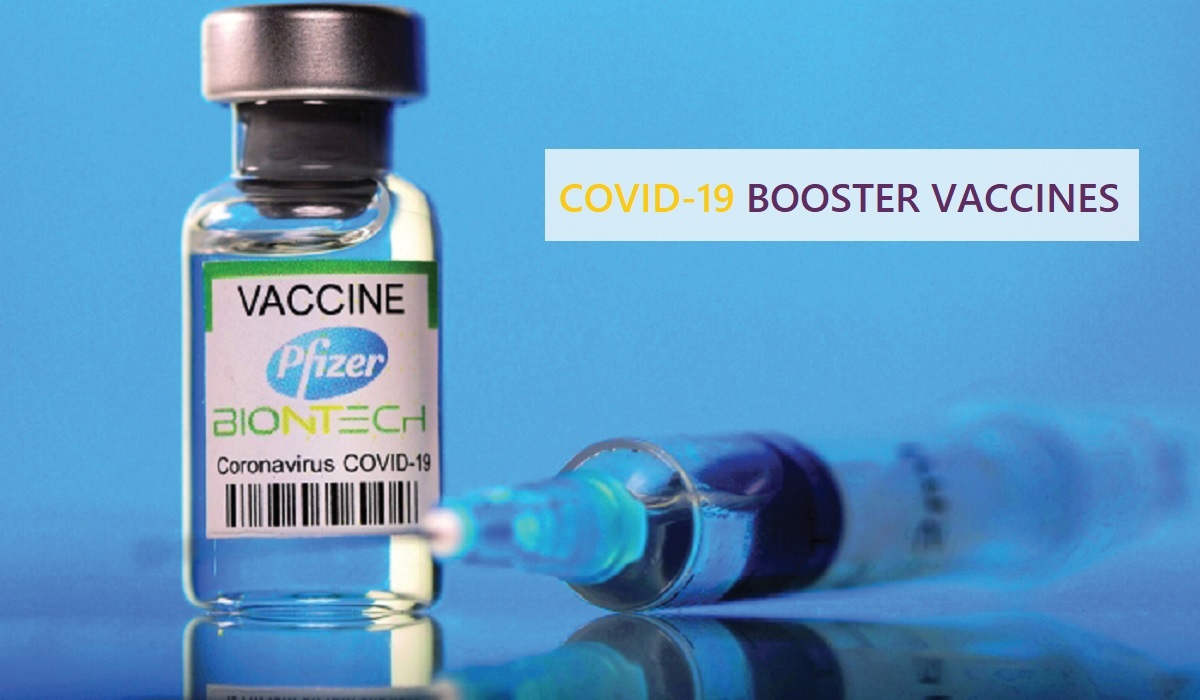
The Ministry of Public Health has approved the administration of Pfizer-BioNTech COVID-19 booster vaccines for children aged 12 to 15 years. The decision is in line with the recent studies that demonstrate the safety and efficacy of booster doses ..
The following is a summary of some recent studies on COVID-19. They include research that warrants further study to corroborate the findings and that has yet to be certified by peer review. Re-infection risk may be higher with Omicron variant S..
The incoming German government wants to make COVID-19 vaccinations mandatory from March 16 for people working in hospitals, nursing homes and other medical practices, according to a copy of draft legislation seen by Reuters on Sunday. Germany has ..
People aged under 30 in Germany should only receive the Biontech/Pfizer (PFE.N)COVID-19 vaccine as it causes fewer heart inflammations in younger people than the Moderna (MRNA.O) shot, an advisory committee said on Wednesday. The committ..
A booster dose of the COVID-19 vaccine developed by Pfizer Inc (PFE.N) and German partner BioNTech SE (22UAy.DE) was 95.6% effective against the coronavirus when compared to a vaccinated group that did not get the third shot, data..
The effectiveness of the Pfizer Inc (PFE.N)/BioNTech SE vaccine in preventing infection by the coronavirus dropped to 47% from 88% six months after the second dose, according to data published on Monday that U.S. health agencies considered when ..
.jpg)
Qatar Secures Place Among the World's Top 10 Wealthiest Nations
.jpg)
Hamad International Airport Witnesses Record Increase in Passenger Traffic

Saudi Arabia: Any visa holder can now perform Umrah

What are Qatar's Labour Laws on Annual Leave?